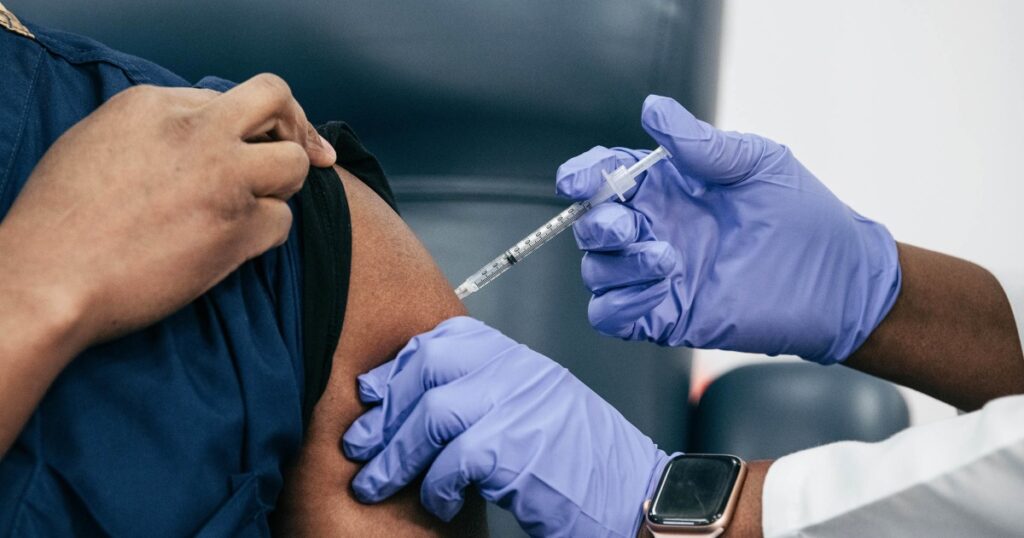
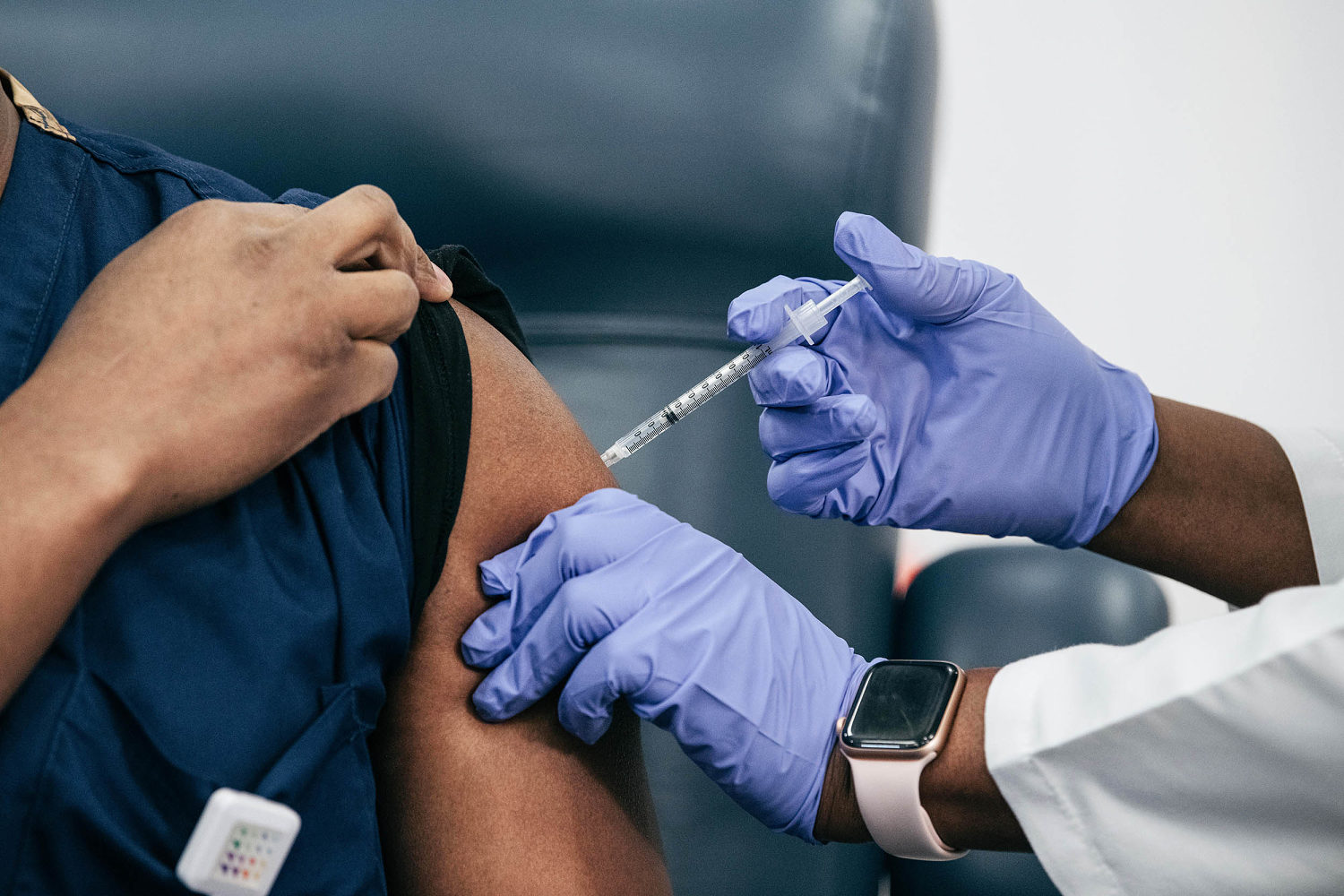
The Food and Drug Administration said late Thursday it would like drugmakers to update the Covid vaccines to target the LP.8.1 strain, fueling concerns that the shots may be limited to only the most at-risk Americans this fall.
The FDA’s decision differs slightly from the recommendation made by its vaccine advisory committee earlier that day, which was to stick to the strains used in the current Covid shots, although panel members said LP.8.1 was a suitable alternative.
Studies by Moderna and Pfizer showed that an LP.8.1-targeted vaccine in fact induced a modestly stronger immune response to the circulating strains than the current Covid shots, which target a variant called JN.1 or one of its descendants, KP.2.
LP.8.1 is also a descendent of JN.1, and it is the dominant strain circulating in the U.S., accounting for roughly 3 in 4 new Covid cases, according to FDA briefing documents released earlier this week. It’s different from a strain dubbed NB.1.8.1 that was recently detected in the U.S. and caused a surge in hospitalizations in China.
Normally, changing the strain for the vaccine wouldn’t raise questions — it’s been the practice of the U.S. in recent years, following a similar model to how the flu shot is updated each year. For Pfizer and Moderna, their mRNA technology makes it particularly easy to update their vaccines.
But this year, the change is expected to trigger new rules by the FDA that new Covid vaccines for healthy kids and adults must undergo placebo-controlled clinical trials — a process Dr. Marty Makary, the agency’s commissioner, told a Senate committee on Thursday could take roughly a year.
The updated Covid shots are still expected to be available in the fall to adults 65 and up and kids and adults with at least one medical condition that puts them at risk for severe illness — two groups that are exempt from the clinical trial requirement.
The list of underlying conditions that raises a person’s risk is extensive — “physical inactivity” is even included. Officials estimated that more than 100 million people in the U.S. would still qualify for a shot.
But the clinical trial requirement “would clearly delay and impede access to vaccines for those people who want it,” said Dr. Jesse Goodman, a professor of medicine and infectious disease at Georgetown University and a former chief scientist at the FDA.
However, Goodman added, a number of questions remain about the FDA’s policy, including how many people would be needed for the trials and whether a new strain selection would indeed trigger the new trial requirement.
When asked by committee members about the new policy on Thursday, FDA officials either said that the questions were off-topic or that the agency was still finalizing the details with drugmakers.
The FDA’s notice also states it would “preferentially” like drugmakers to update their shots to the LP.8.1 strain — potentially leaving room for drugmakers to stick to their existing formulations.
“I suspect we will learn more and their approach may become clearer and/or evolve in coming days,” Goodman said. He added it makes sense to leave room for drugmakers to update to either strain, noting it’s often difficult to predict how Covid will evolve.
The FDA directed all media inquiries to the Department of Health and Human Services.
“The COVID-19 public health emergency has officially ended, and we are entering a new phase in our response to the virus,” Emily Hilliard, a spokesperson for HHS, wrote in an emailed statement. “A rubber-stamping approach to approving COVID boosters in perpetuity without updated clinical trial data under the Biden Administration is now over.”
In a statement, a Pfizer spokesperson directed NBC News to comments by the company Thursday, which stated it was prepared to “initiate supply of a 2025/2026 vaccine formula per FDA guidance, immediately upon approval.”
Moderna and Novavax did not immediately respond to requests for comment.
There are also questions about insurance coverage and whether patients who are ineligible for the shots would have to pay out of pocket if they wanted one.
Pfizer and Moderna are charging up to $150 per dose for a Covid vaccine, according to the CDC’s vaccine price list. The agency doesn’t list the cost of the Novavax vaccine, which was fully approved earlier this month.
In a statement, a spokesperson for America’s Health Insurance Plans, an industry trade group, said, “Health plans have prioritized providing affordable access to preventive services, including vaccines.”
“We will continue to monitor the forthcoming recommendations and guidance from ACIP and CDC,” the spokesperson said, referring to the CDC’s vaccine advisory committee. “As of today, there is no change in how plans cover the existing vaccines for the previously recommended populations.”
 Latest Breaking News Online News Portal
Latest Breaking News Online News Portal






